I-Aqua
Home / By Brand Name / I-Aqua
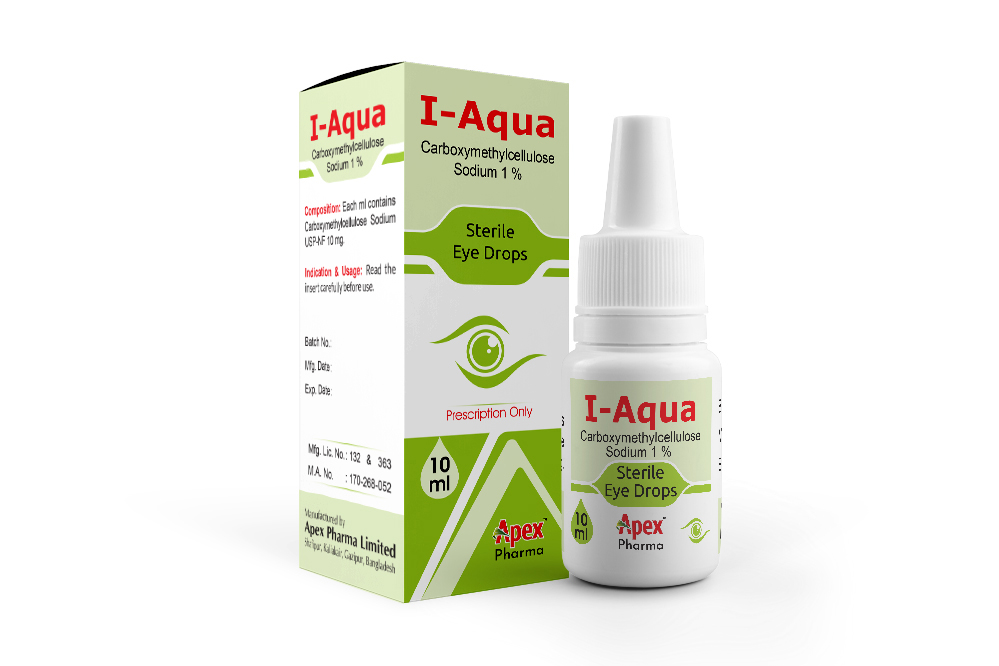
I-Aqua
Generic Name: Carboxymethylcellulose Sodium
Therapeutic Class: Artificial Tear
COMPOSITION
I-Aqua: Each ml contains Carboxymethylcellulose Sodium USP-NF 10 mg.
DESCRIPTION
I-Aqua Eye Drops is a sterile lubricant Eye Drops. The higher strength of Carboxymethylcellulose Sodium USP-NF provides long-lasting relief for dryness by forming a soothing gel after administration of the drops on to the eyes. It contains oxidative preservative Stabilized Oxychloro Complex which breaks down into the natural components of tears (water, oxygen, sodium & chloride salts).
INDICATION
I-Aqua Eye Drops is indicated for the long-lasting relief of burning, irritation, and discomfort due to dryness of the eye or exposure to wind or sun.
DOSAGE AND ADMINISTRATION
Instill 1 or 2 drops in the affected eye(s) as needed or as directed by physician.
CONTRAINDICATION
I-Aqua is contraindicated in patients with known hypersensitivity to any ingredient of this formulation.
WARNING & PRECAUTION
For external use only. Do not use if the color of the solution changes or the solution becomes cloudy.
Discontinue use and consult a physician if eye pain, changes in vision, continued redness or irritation of the eye are experienced or if the condition worsens or persists for more than 72 hrs.
DRUG INTERACTIONS
Concomitant ocular medications should be administered at least 5 min apart from the instillations of this Eye Drops to avoid washout effects.
USE IN PREGNANCY AND LACTATION
Pregnancy: There are no data on the use of Carboxymethylcellulose Sodium 1% during pregnancy and
lactation in humans. Animal studies did not show harmful effects with the active ingredient,
Carboxymethylcellulose Sodium.
Lactation: Carboxymethylcellulose Sodium is not absorbed systemically; there is no known potential for excretion in human breast milk.
PEDIATRIC USE
The safety and effectiveness in pediatric patients have not been established.
GERIATRIC USE
No overall differences in safety or effectiveness have been observed between elderly and other adult patients.
SIDE EFFECTS
Visual disturbances, ocular discharge and eye pruritus are common adverse drug reactions were reported with Carboxymethylcellulose Sodium. Conjunctival hyperaemia and eyelid edema are reported but the frequency is very less.
Storage ConditionsStore below 300 C in a cool and dry place protected from light. Keep all medicine out of reach of children. Solution can be used up to 30 days after first opening.
PRODUCTS